On this week’s episode, we’re continuing our Guidelines Series exploring the 2022 ESC/ERS Guidelines for the diagnosis and treatment of Pulmonary Hypertension. If you missed our first episode in the series, give it a listen to hear about the most recent recommendations regarding Pulmonary Hypertension definitions, screening, and diagnostics. Today, we’re talking about the next steps after diagnosis. Specifically, we’ll be discussing risk stratification, establishing treatment goals, and metrics for re-evaluation. We’ll additionally introduce the mainstays of pharmacologic therapy for Pulmonary Hypertension.

Meet Our Co-Hosts
Rupali Sood grew up in Las Vegas, Nevada and made her way over to Baltimore for medical school at Johns Hopkins. She then completed her internal medicine residency training at Massachusetts General Hospital before returning back to Johns Hopkins, where she is currently a pulmonary and critical care medicine fellow. Rupali’s interests include interstitial lung disease, particularly as related to oncologic drugs, and bedside medical education.
Tom Di Vitantonio is originally from New Jersey and attended medical school at Rutgers, New Jersey Medical School in Newark. He then completed his internal medicine residency at Weill Cornell, where he also served as a chief resident. He currently is a pulmonary and critical care medicine fellow at Johns Hopkins, and he’s passionate about caring for critically ill patients, how we approach the management of pulmonary embolism, and also about medical education of trainees to help them be more confident and patient centered.
Key Learning Points
1) Episode Roadmap
- How to set treatment goals, assess symptom burden, and risk-stratify patients with suspected/confirmed pulmonary arterial hypertension (PAH).
- What tools to use to re-evaluate patients on treatment
- Intro to major PAH medication classes and how they map to pathways.
2) Case-based diagnostic reasoning
Patient: 37-year-old woman with exertional dyspnea, mild edema, abnormal echo, telangiectasias + epistaxis → raises suspicion for HHT (hereditary hemorrhagic telangiectasia) and/or early connective tissue disease.
- Key reasoning move: start broad (Groups 2–5) and narrow using history/exam/testing.
- In a young patient without obvious left heart or lung disease, think more about Group 1 PAH (idiopathic/heritable/associated).
HHT teaching point: HHT can cause PH in more than one way:
- More common: high-output PH from AVMs (often hepatic/pulmonary)
- Rare (1–2% mentioned): true PAH phenotype (vascular remodeling; associated with ALK1 in some patients), behaving like Group 1 PAH.
3) Functional class assessment
WHO Functional Class:
- Class I: no symptoms with ordinary activity, only with exertion
- Class II: symptoms with ordinary activity
- Class III: symptoms with less-than-ordinary activity (can’t do usual chores/shopping without dyspnea)
- Class IV: symptoms at rest
Practical bedside tip they give:
- Ask if the patient can walk at their own pace or keep up with a similar-age peer/partner. If not, think Class II (or worse).
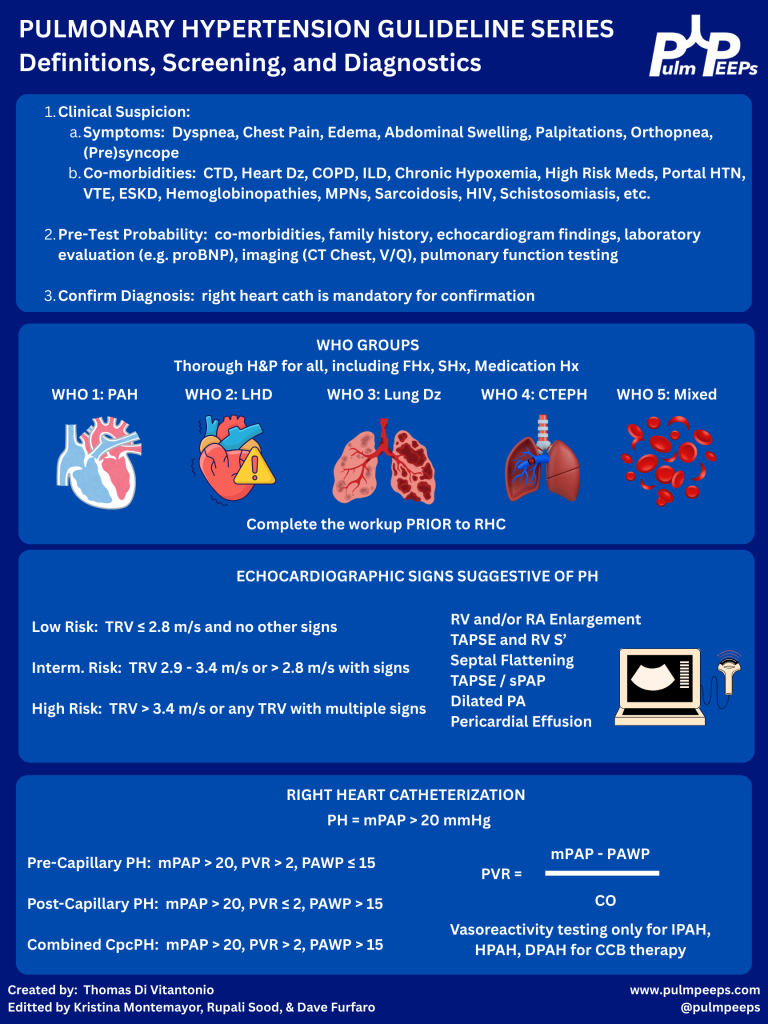
4) Risk stratification at diagnosis: why, how, and which tools
Big principle: treatment choices are driven by risk, and the goal is to move patients to low-risk quickly.
ESC/ERS approach at diagnosis (as described):
- Use a 3-strata model predicting 1-year mortality:
- Low: <5%
- Intermediate: 5–20%
- High: >20%
ESC/ERS risk assessment variables (10 domains discussed):
- Clinical progression, signs of right heart failure, syncope
- WHO FC
- Biomarkers (NT-proBNP)
- Exercise capacity (6MWD)
- Hemodynamics
- Imaging (echo; sometimes cardiac MRI)
- CPET (peak VO₂; VE/VCO₂ slope)
They note: even if you don’t have everything, the calculator can still be useful with ≥3 variables.
REVEAL 2.0:
- Builds on similar core variables but adds further patient context (demographics, renal function, BP, DLCO, etc.)
Case result: both tools put her in intermediate risk (ESC/ERS ~1.6; REVEAL 2.0 score 8), underscoring that mild symptoms can still equal meaningful mortality risk.
5) Treatment goals and follow-up philosophy
What they explicitly prioritize:
- Help patients feel better, live longer, and stay out of the hospital
- Use risk tools to communicate prognosis and to track improvement
- Reassess frequently (they mention ~every 3 months early on) until low risk is achieved
- “Time-to-low-risk” is an important treatment goal
Also emphasized:
- The diagnosis is psychologically heavy; patients need clear counseling, reassurance about the plan, and connection to support groups.
6) Medication classes for the treatment of PAH
Nitric oxide–cGMP pathway
- PDE5 inhibitors: sildenafil, tadalafil
- Soluble guanylate cyclase stimulator: riociguat
- Important safety point: don’t combine PDE5 inhibitors with riociguat (risk of significant hypotension/hemodynamic effects)
Endothelin receptor antagonists (ERAs)
- “-sentan” drugs: bosentan (less used due to side effects/interactions), ambrisentan, macitentan
- Teratogenicity emphasized
- Hepatotoxicity that requires LFT monitoring
- Can cause fluid retention and peripheral edema
Prostacyclin pathway
- Prostacyclin analogs/agonists:
- Epoprostenol (potent; short half-life; IV administration)
- Treprostinil (IV/SubQ/oral/inhaled options)
- Selexipag (oral prostacyclin receptor agonist)
7) Sotatercept (post-guidelines)
They note sotatercept wasn’t in 2022 ESC/ERS but is now “a game changer” in practice:
- Mechanism: ligand trap affecting TGF-β signaling / remodeling biology
- Positioned as potentially more disease-modifying than pure vasodilators
- Still evolving: where to place it earlier vs later in regimens is an active question in the field
8) How risk category maps to initial treatment intensity
General approach they outline:
- High risk at diagnosis: parenteral prostacyclin (IV/SubQ) strongly favored, often aggressive early
- Intermediate risk: at least dual oral therapy (typically PDE5i + ERA); escalate if not achieving low risk
- Low risk: at least one oral agent; many still use dual oral depending on etiology/trajectory
For the case: intermediate-risk → start dual oral therapy (they mention tadalafil + ambrisentan as a typical choice), reassess in ~3 months; add a third agent (e.g., selexipag/prostacyclin pathway) if not low risk.
References and Further Reading
Podcast: Play in new window | Download
Subscribe: Apple Podcasts | Spotify | Amazon Music | Android | iHeartRadio | Podcast Index | RSS | More