For today’s podcast we have a special episode. We were extremely grateful to be invited to present live at CHEST 2025 this year. Kristina Montemayor, and Pulm PEEPs Associate Editors Luke Hedrick, Tom Di Vitantonio, and Rupali Sood hosted a session entitled “Widened Airways and Narrowed Differentials”. It is a great session around bronchiectasis. Enjoy!

Meet Our Guests
Dr. Doreen Addrizzo-Harris is a Professor of Medicine at NYU where she is also Associate Director of Clinical and Academic Affairs for the pulmonary and critical care division. In addition to that, she’s the director of the bronchiectasis and NTM program and also serves as a program director for the pulmonary and critical care fellowship.
Case Snapshot
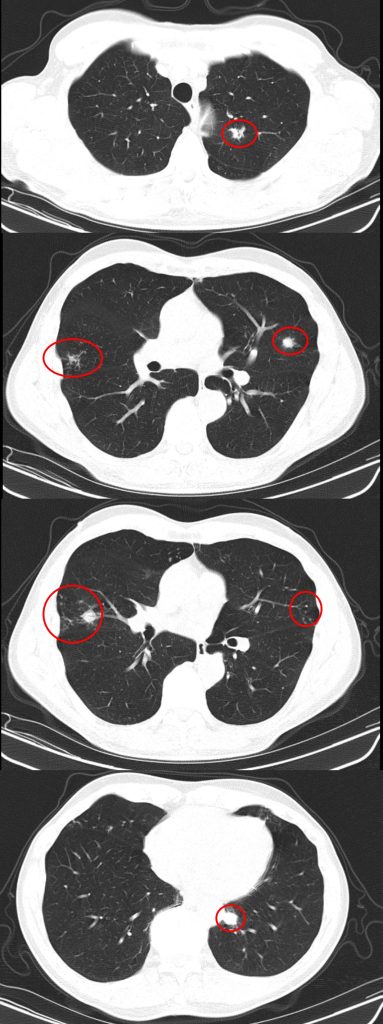
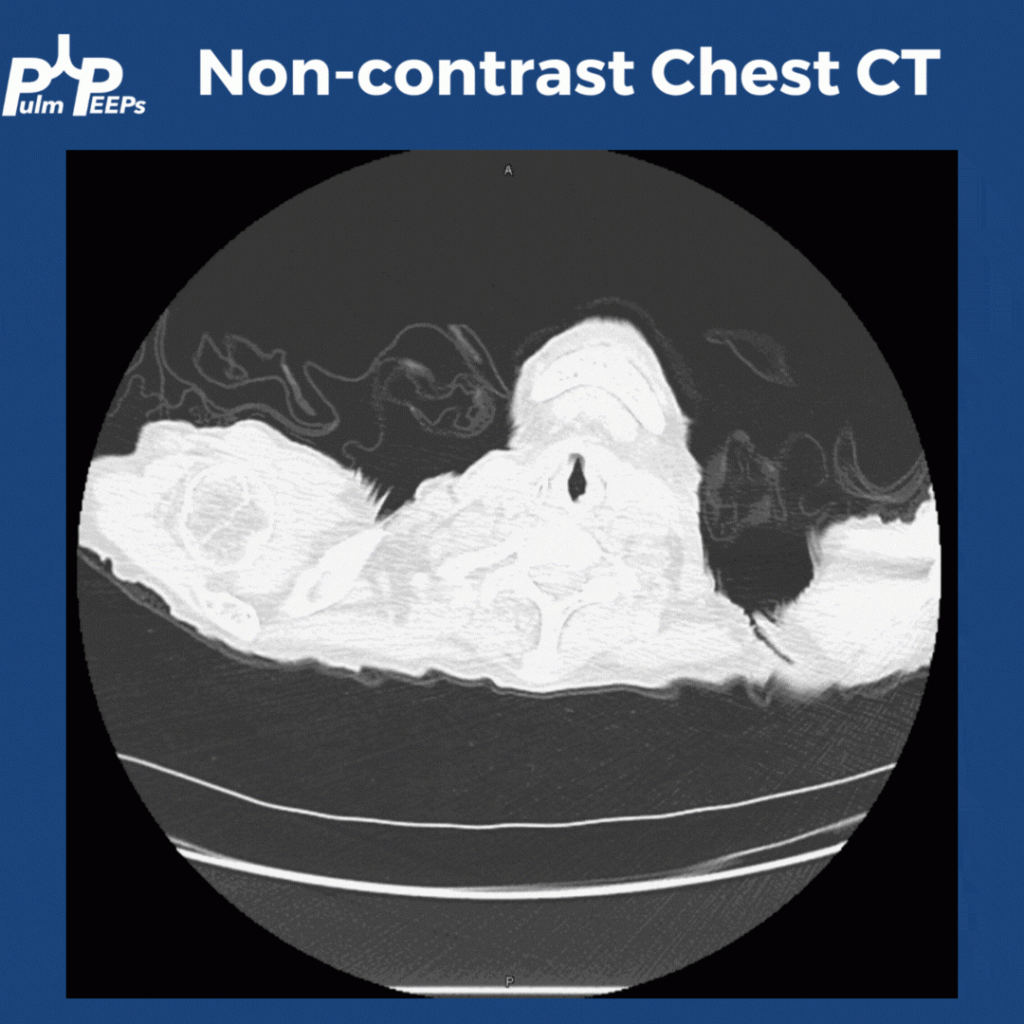
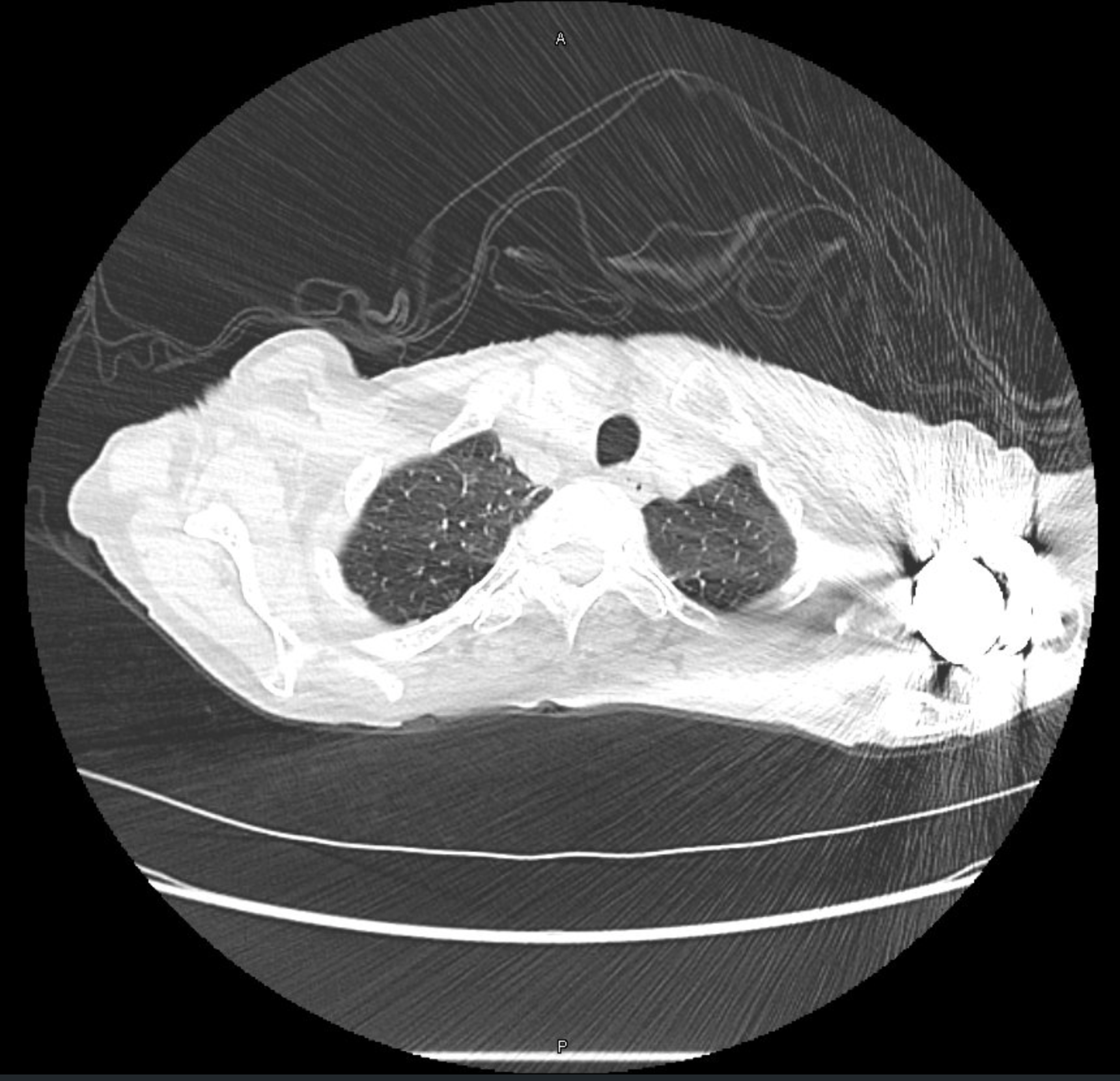
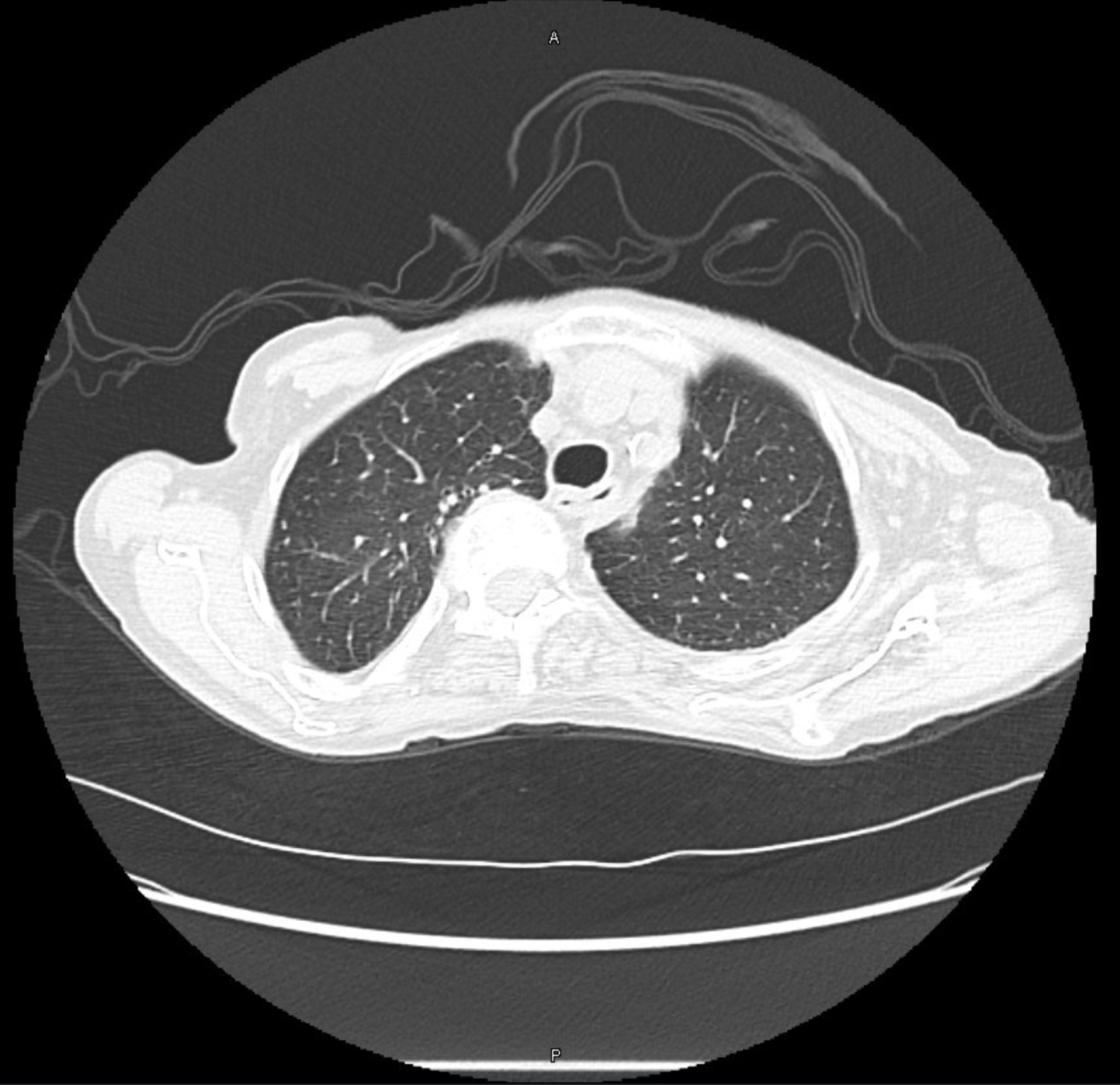
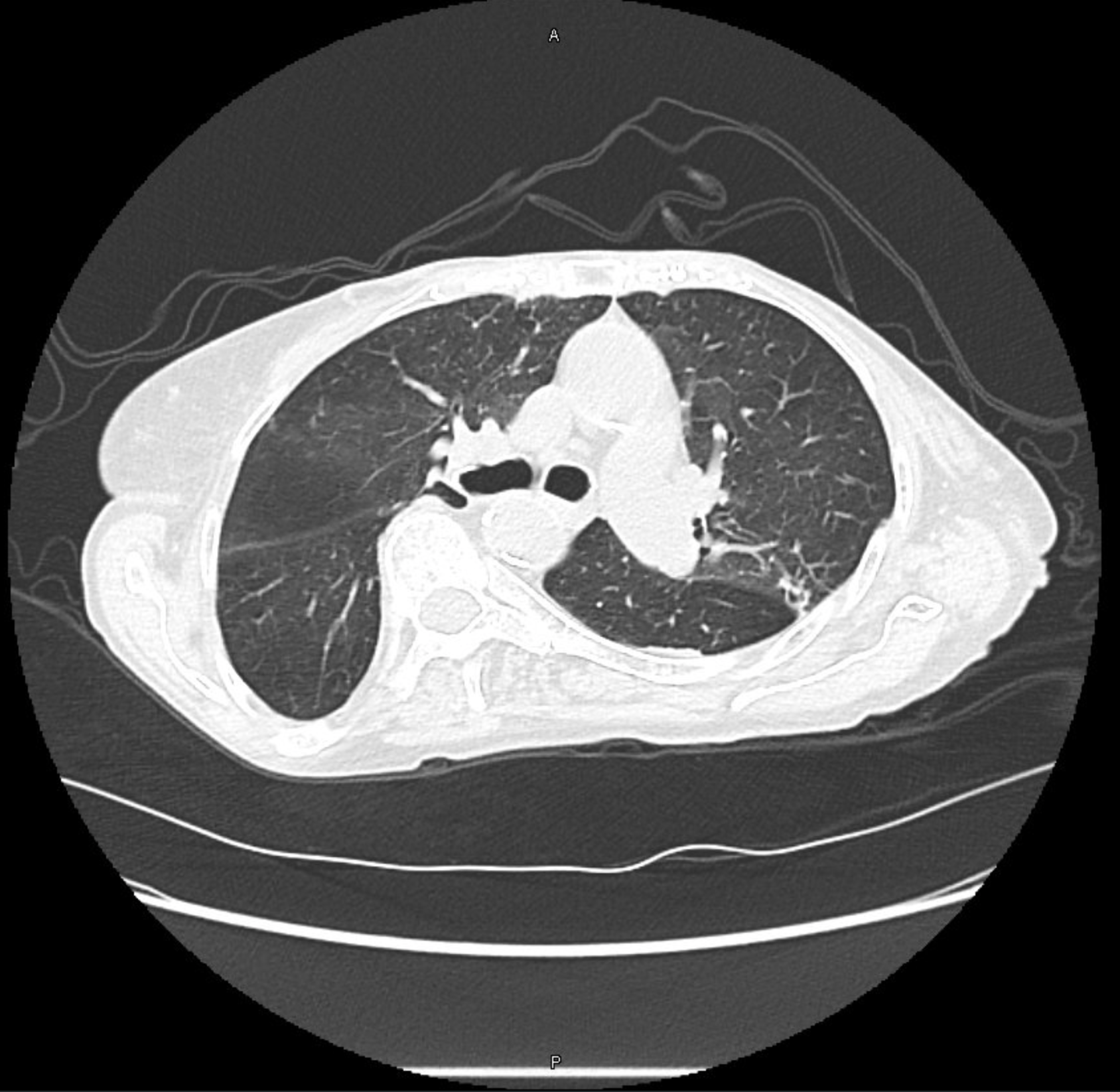
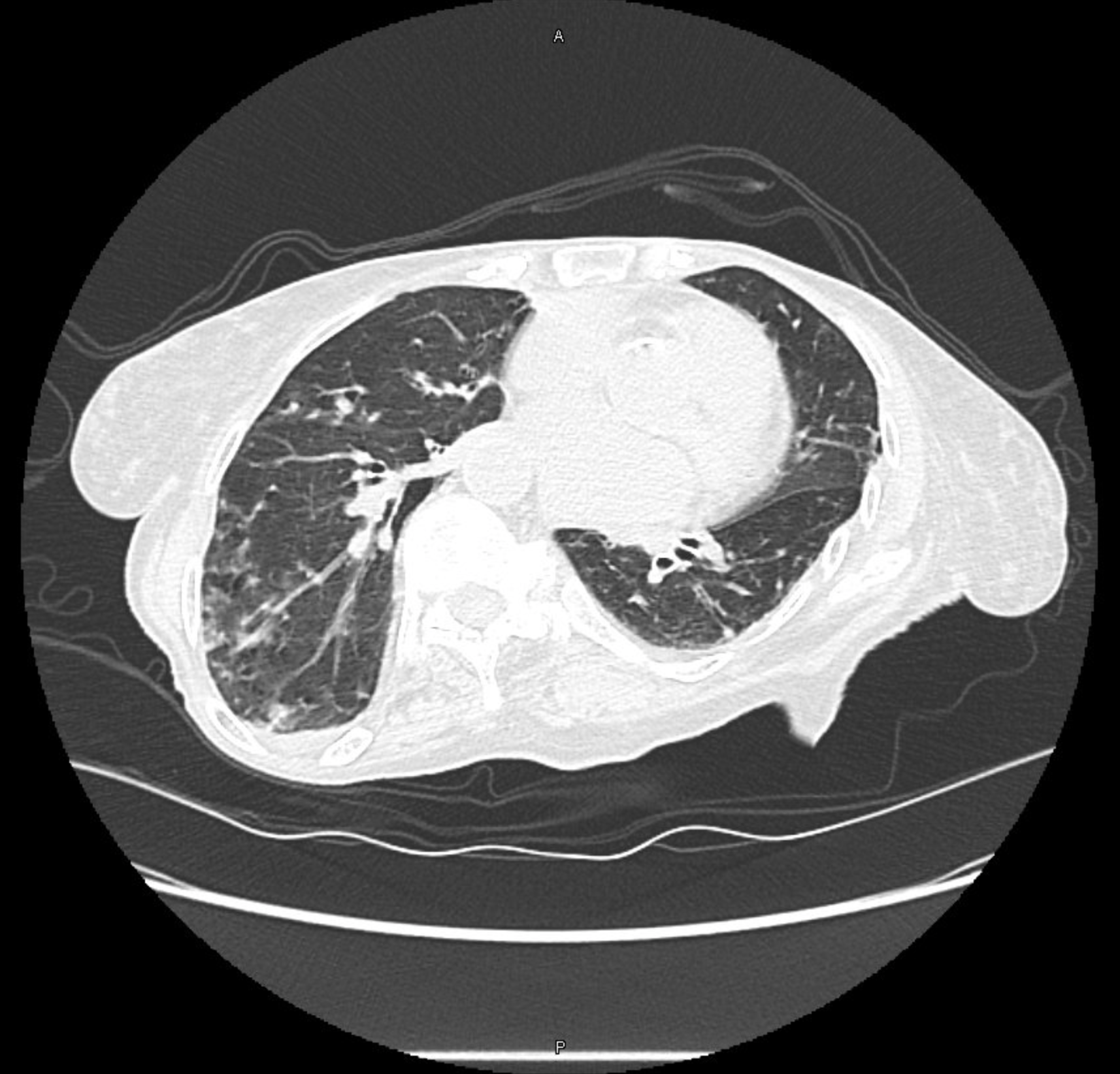
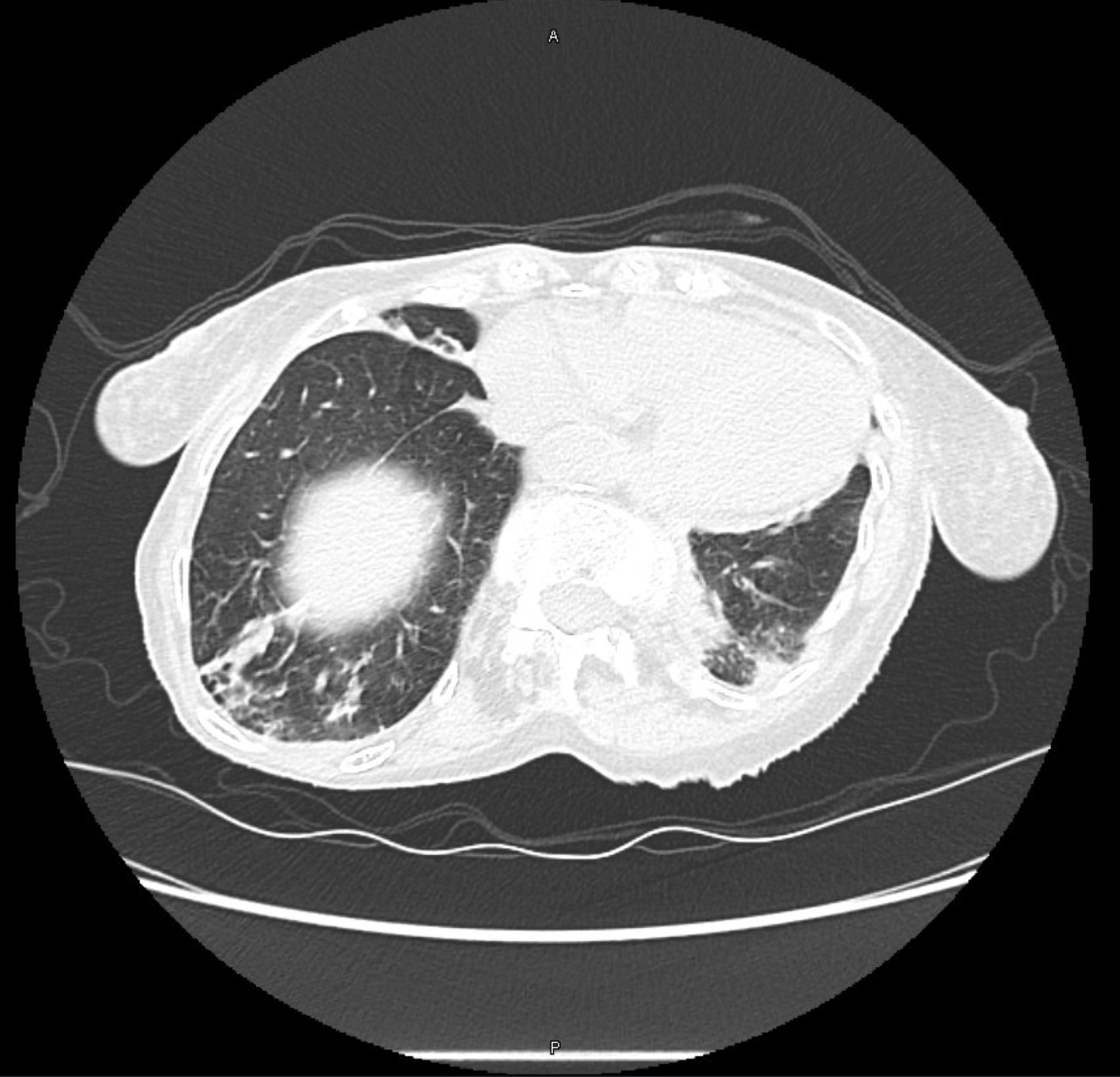
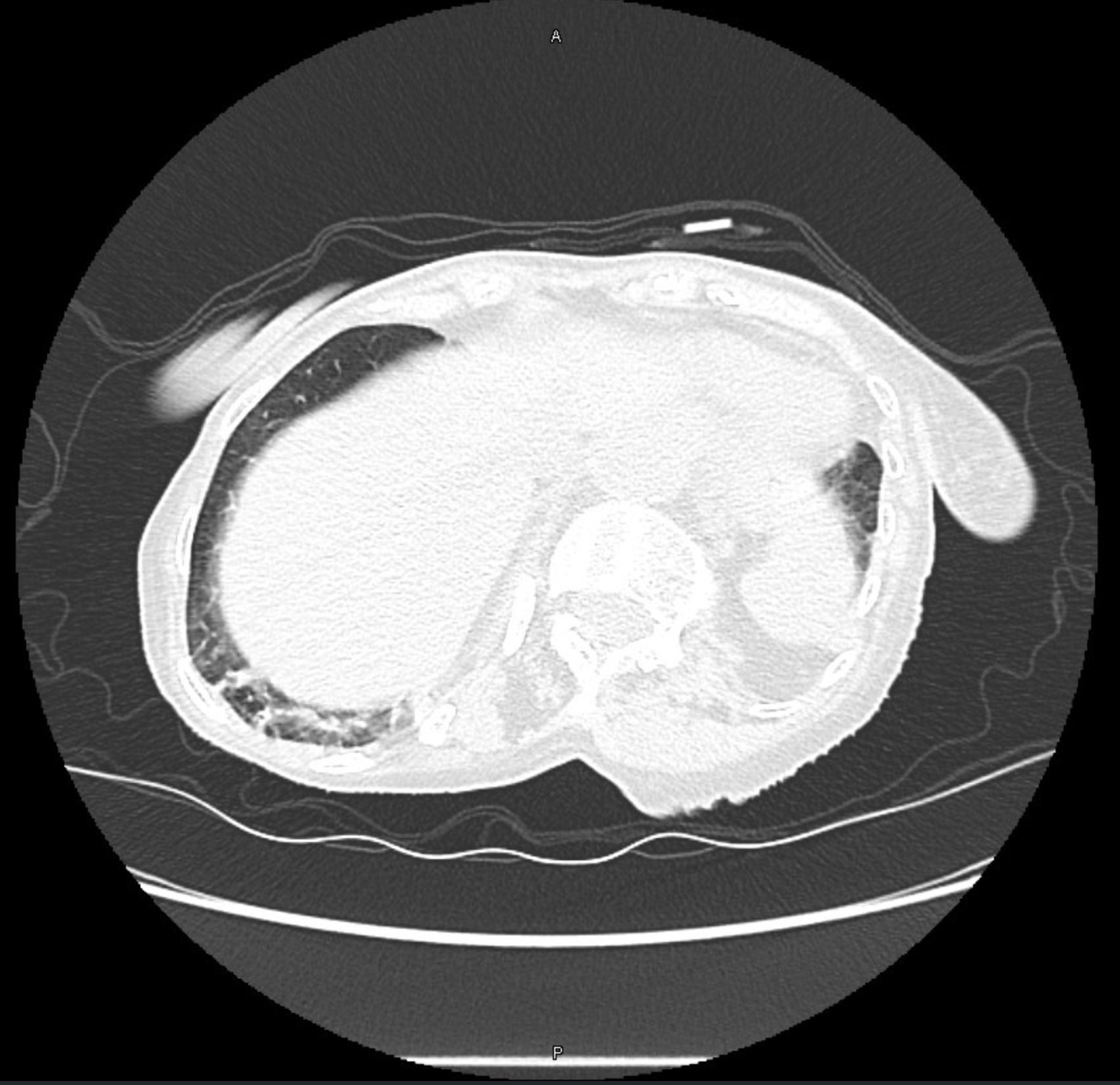
60-year-old with CLL (in remission) → recurrent “pneumonias,” diffuse (not single-lobe), later dx’d with CVID; serial CTs: upper-lobe–predominant bronchiectasis, tree-in-bud, mucus impaction; multiple AFB+ cultures (MAC, later M. abscessus); recurrent bacterial flares (MSSA/MRSA).
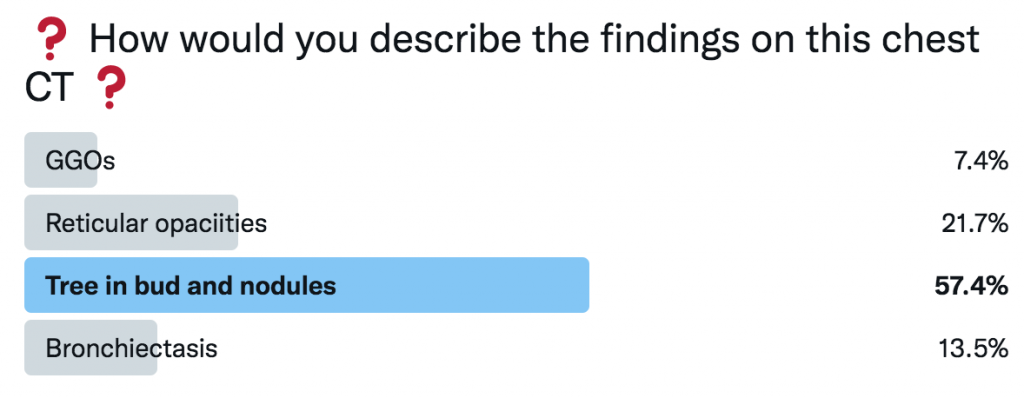
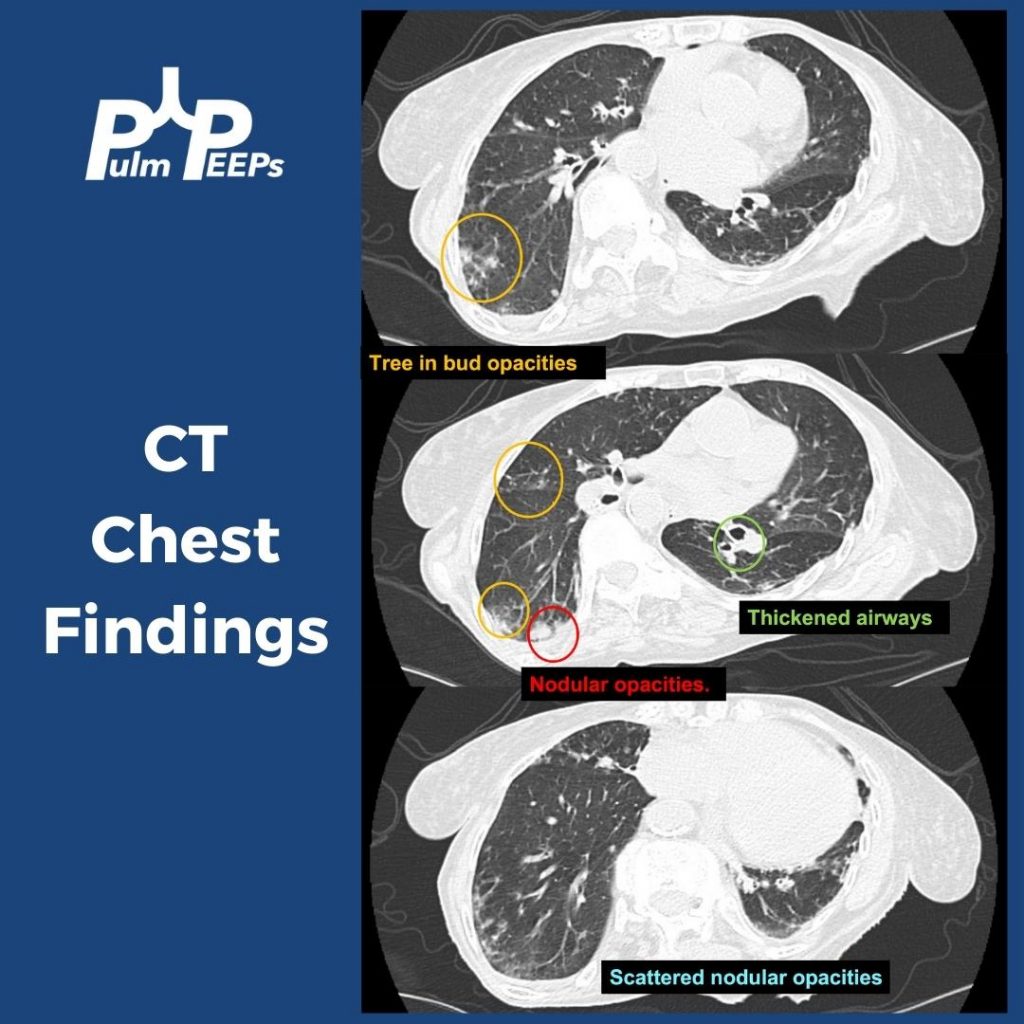
CT Images

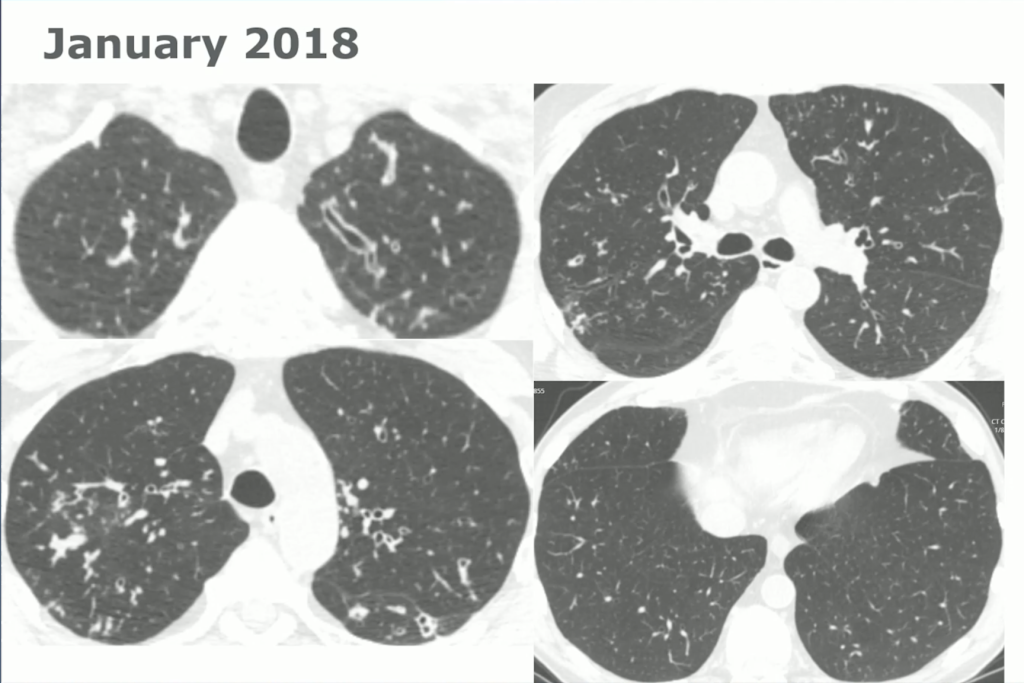
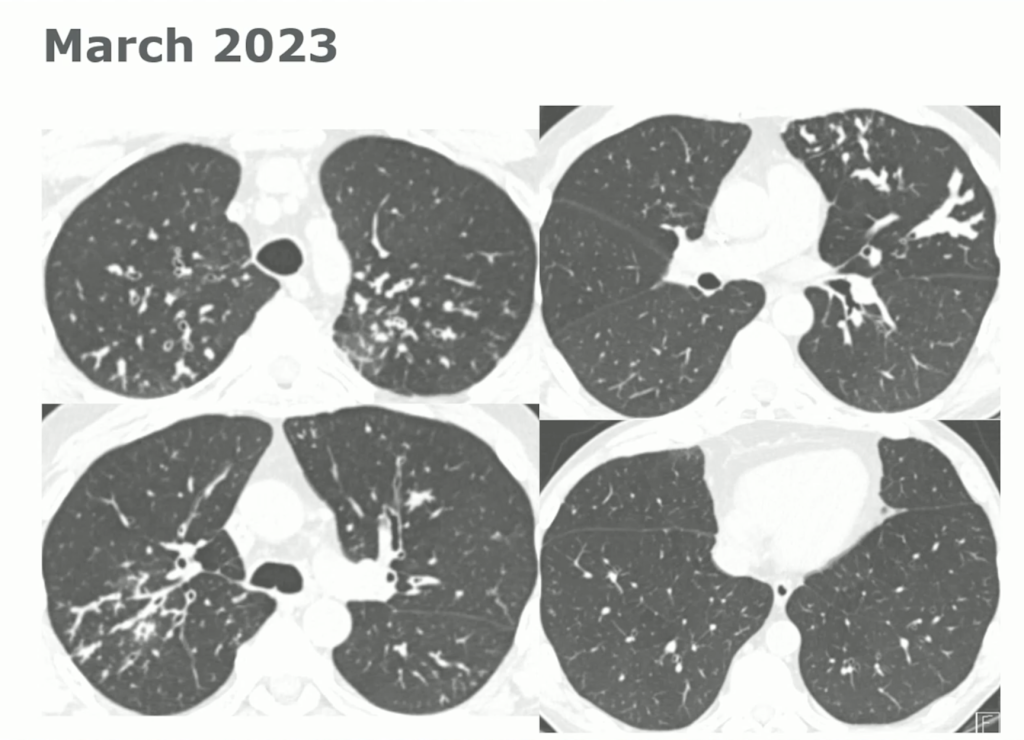
Key Learning Points
Imaging pearls
- Tree-in-bud = small airways (bronchiolar) impaction/inflammation, not a diagnosis. Differential guided by distribution + chronicity:
- Acute/diffuse → bacterial/viral/NTM infection
- Dependent/basal → aspiration
- Persistent + nodular + bronchiectasis → NTM common
- Bronchiectasis CT signs (think: “ring, taper, edge”):
- Broncho-arterial ratio >1 (signet-ring)
- Lack of normal tapering
- Visible bronchi within 1 cm of pleura
- Location matters:
- Upper lobes → CF, sarcoid, prior TB/radiation
- Middle lobe/lingula → NTM classic; consider ABPA if central
- Lower lobes → aspiration, PCD, CTD, immunodeficiency
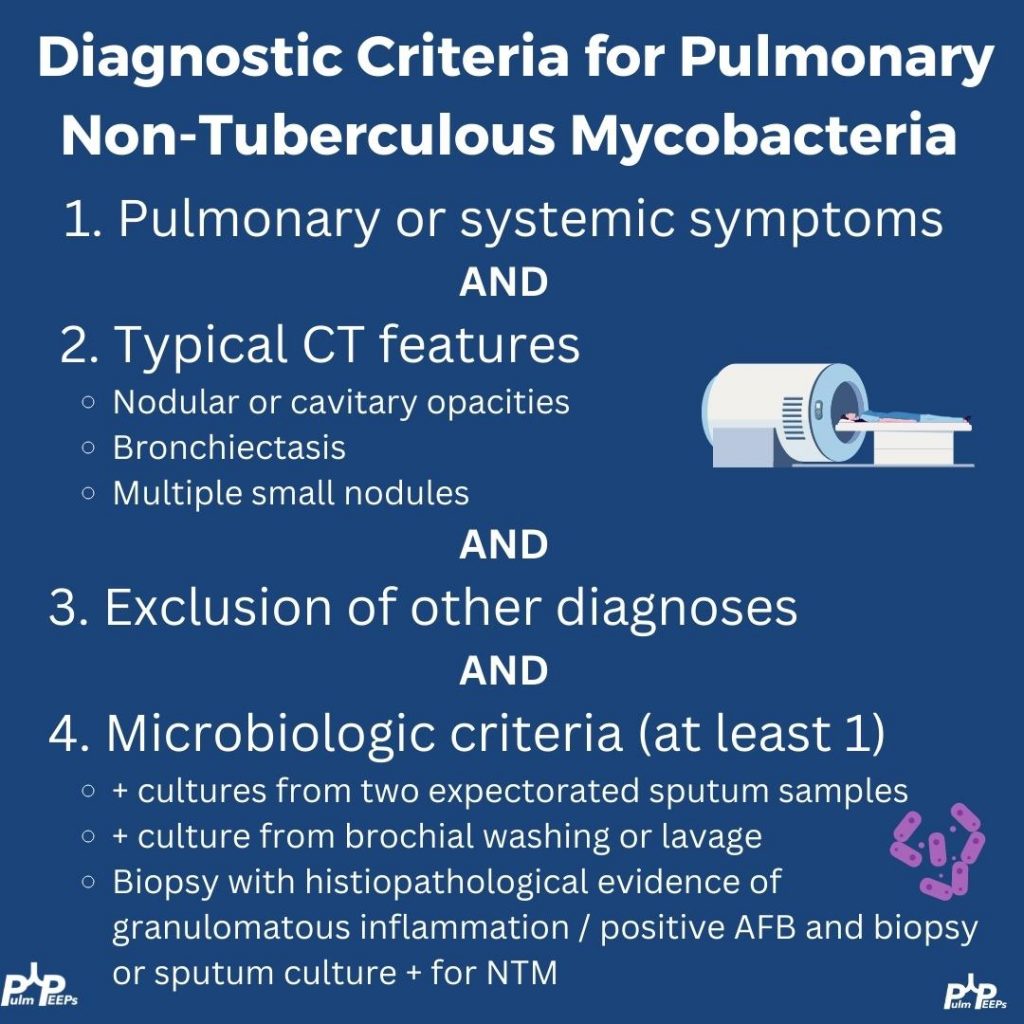
NTM: diagnosis & when to treat
- Use all three (2020 guideline frame): clinical symptoms, compatible CT, microbiology (≥2 sputum cultures or 1 bronch +, etc.).
- Not every positive culture = disease needing drugs. If you defer pharmacologic therapy, follow closely (symptoms, sputum, PFTs, interval CT if change).
- Bug matters: MAC, M. abscessus, kansasii etc. “Low-virulence” species (e.g., M. gordonae) can still flag underlying airway disease.
- Regimens (MAC, macrolide-susceptible): azithro + ethambutol + rifampin (intermittent for nodular-bronchiectatic; daily ± IV amikacin for fibro-cavitary/advanced).
- Macrolide is the backbone; the others protect against resistance.
- M. abscessus: check for inducible macrolide resistance (prolonged in-vitro testing).
- Monitoring: sputum q1–3 mo; labs (CBC/CMP), vision (ethambutol), hearing (aminoglycosides). Treat ~12 months beyond culture conversion.
- Anti-inflammatory macrolide for bronchiectasis is contraindicated if macrolide-susceptible NTM is present—risk of resistance.
Bronchiectasis management essentials
- It’s a syndrome: symptoms/exacerbations plus CT changes.
- Airway clearance is foundational (exercise + devices ± hypertonic saline/DNase when indicated). Expect CT and symptom gains with adherence.
- Exacerbations often need ~14 days of pathogen-directed antibiotics (short courses may fail). Take the “easy win” when a conventional pathogen explains the flare.
Workup framework (start with a core bundle, then target)
Core “every patient” bundle
- CBC with diff (look for eosinophilia/hematologic clues)
- Quantitative IgG/IgA/IgM (primary/secondary immunodeficiency)
- ABPA screen: total IgE + Aspergillus-specific IgE/IgG
- Sputum cultures: routine bacteria + AFB + fungal (if producing)
- Baseline PFTs
Targeted tests (guided by history, distribution, microbes)
- CF evaluation: sweat chloride and/or CFTR genotyping (especially with upper-lobe disease, chronic sinusitis/nasal polyps, pancreatitis/malabsorption, infertility/CAVD).
- PCD: nasal NO, genetics, specialized ciliary studies (adult cases may be mild and missed by genetics alone).
- Alpha-1 antitrypsin (never-smoker emphysema, liver hx)
- CTD serologies (RA, Sjögren’s, etc.), if suggestive
- Aspiration/upper-GI assessment when basal-predominant or reflux symptoms
- For suspected/known CVID: vaccine response assessment if not on replacement (this patient was already on IVIG).
Practical diagnostic habits
- Re-read the CT yourself—radiology may under-call mild bronchiectasis in ED/PE-protocol scans.
- Use a diagnostic time-out when the course isn’t fitting: name your working dx, list fits/mismatches, consider common diseases with atypical presentations, multi-morbidity, and can’t-miss alternatives; ask for help early; communicate uncertainty.
Teach-to-remember pearls from the case
- Recurrent, geographically scattered pneumonias → think systemic causes (immunodeficiency, CF/PCD), not just focal anatomic problems.
- Upper-lobe bronchiectasis + CAVD is a CF red flag—even in the 60s. Adult-onset CF is real and actionable.
- In CF today, MSSA can be more common than Pseudomonas on culture; don’t let absence of Pseudomonas dissuade you.
- Airway clearance adherence can change CTs; instruct patients to ramp up before surveillance scans for a fair assessment.
- If symptoms abate with targeted therapy to a conventional pathogen, you may avoid immediate NTM re-treatment—but keep a tight follow-up loop.
Podcast: Play in new window | Download
Subscribe: Apple Podcasts | Spotify | Amazon Music | Android | iHeartRadio | Podcast Index | RSS